
cormeo: Smart Information Lifecycles for Life Sciences
We build the trusted bridge between industry, authorities, healthcare professionals, and patients.
Overcome the challenges in creating, managing, and distributing regulated information - enter the Smart Information Lifecycle.
At cormeo, we revolutionize how regulated information is created, managed, and distributed in the life sciences sector. By uniting the expertise of Docuvera, EXTEDO, medicines.ie and Rote Liste, we deliver a seamless, end-to-end approach - a Smart Information Lifecycle - that empowers life sciences companies, authorities, and healthcare professionals to efficiently navigate the complex landscape of medical and regulatory information.
AI + Structured Content Authoring
Regulatory, Quality & Safety Information Management
Medicines Information Distribution
Our Goal: A Smart Information Lifecycle
Whom We Connect
At cormeo, we ensure a seamless and compliant flow of trusted medicinal product information, connecting all key stakeholders with accuracy, transparency, and ease of access.
How We Do It
Imagine a seamless end-to-end solution suite that enables you to create, compile, manage, exchange, and distribute regulated information.
Structured components, master data and submissions are led back and forth through streamlined workflows until a successful approval by the health agency. Once on the market, healthcare professionals and patients access the medicinal product information to choose the best therapy – anytime, on any device.
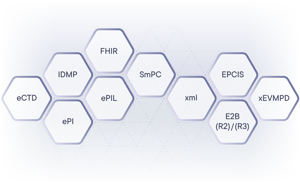
Shaping The Regulations We Support
Our long-standing commitment to delivering the best solutions to our customers and our engagement within the overall regulatory environment allowed us to establish robust connections to international experts and decision-makers.
We are involved in the creation and deployment of (inter-)national standards and are thus always up-to-date when it comes to their integration into our customers’ daily tasks.
Standards We Support:
eCTD | IDMP | ePI | FHIR | ePIL | SmPC | xEVMPD | E2B (R2) / (R3) | xml | EPCIS | SRD | ATC | DHPC

Introducing cormeo
How You Benefit From Our Solutions

Healthcare Professionals and Patients
With cormeo’s innovative solution suite, doctors, pharmacists, and patients receive clear, accurate, and timely information, enabling them to leverage the best possible care. Our end-to-end process from lab to patient is faster, safer, and more efficient than ever before.
Health Authorities
cormeo’s solutions streamline the assessment process for regulatory authorities. By providing a centralized repository for all master data and enabling smart data exchanges, we help authorities ensure compliance, improve data accuracy, and facilitate faster approval times. Regulatory workflows become more efficient and public health management improves significantly.
Life Sciences Companies
The unique cormeo approach enables fast collaboration with agencies, precise communication with healthcare professionals, and streamlined information exchange among stakeholders. Organizations can manage master data in a single source of truth, ensuring consistency and reliability. Additionally, analyzing information usage data enables smart market decisions, driving growth and innovation.
This comprehensive approach reduces time-to-market, ensures compliance with global standards, and enables effective responses to regulatory changes.
/Event%20Logos/DIA.png)

